Water Quality Testing – Otter Lake
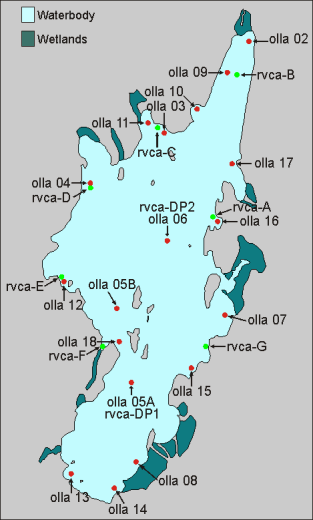
Water quality testing is an important diagnostic tool to help residents of Otter Lake monitor the health of the lake. The ecological and trophic status of a lake is generally determined by the levels of nutrients it contains and these are what we (OLLA) and RVCA measure at different sites around the lake at least three or four times a year. OLLA and RVCA’s test sites are shown on the map on the right. The sites have been chosen to be representative of the whole lake. Sites 05A, O5B and 06 represent the three deepest water sites (more than 90ft). Sites 04, 07, 08, 11 and 18 are in areas where there are known inflows from streams and wetlands into the lake. Other sites are in shallow bays where there is an increased tendency for weed and algae growth.
An overview of Factors that Influencing Water Quality
Water Clarity
Recreational water quality can be expressed in terms of how clear the water appears. Water clarity is influenced by the amount of soil sediment and phytoplankton, or microscopic algae that are present in the water. Clarity is measured by a simple visual test using a Secchi Disk, 20-centimeter black and white disk attached to a measured line. The disk is then lowered into the lake until it is no longer visible and the depth recorded.
Nutrients & Bacteria
Information on water quality is gained through analysis of samples for nutrients, specifically phosphorus and nitrogen, which gives an indication of how much nutrient and energy is available for the growth of algae and aquatic plants.
- Nitrogen is an important and essential nutrient in aquatic ecosystems. In addition to fertilizers, agricultural waste and wastewater contribute nitrogen into lakes. In large amounts, ammonia and nitrates can be toxic to aquatic organisms. Total Kjeldahl Nitrogen (TKN) determines the concentration of all forms of nitrogen in the lake. While there currently are no guidelines for acceptable levels of TKN, according to RVCA, TKN in water bodies not influenced by excessive organic inputs typically range from 100 to 500 µg/L.
- Phosphorous is generally recognized as the limiting nutrient in freshwater ecosystems and the major nutrient contributing to eutrophication in lakes. Since phosphorous is the principal source of energy for all living organisms the amount of phosphorous in the environment will determine how fast an organism grows and proliferates. Phosphorus is therefore the principal limiting factor in the growth of algae. Phosphorus levels below 5 µg/L are typical of oligotrophic lakes that generally are clear and deep with few nutrients. Such lakes are typically found in the northern regions of Ontario. Phosphorous levels above 20 µg/L are typical of eutrophic lakes that are laden with nutrients which lead to excessive algae and plant growth. Mesotrophic lakes are in between these two extremes and are typical of the lakes found in our region of Ontario. Otter Lake is classified as an oligotrophic lake.
- Bacteria are present in all lakes. They will be found in the feces of the wildlife (fish, waterfowl, beavers, etc.) that inhabit the lake and are usually present in soil. Most are not harmful to humans, however some, such as Escherichia coli (E. Coli) do produce pathogenic toxins. Therefore, levels of E. Coli are often used as indicators of possible contamination by fecal matter. Thus, high E. Coli levels in lakes or rivers can be an indication of septic pollution. The recommended level of E. Coli in a lake for recreational safety is less than 100 colony-forming units (cfu) per 100ml of water. E.coli at any level is unacceptable for drinking water.
- Dissolved oxygen (DO) is a measure of how much oxygen is dissolved in the water and the amount of oxygen available to living aquatic organisms. Without DO a lake would be totally without any aquatic life. The amount of dissolved oxygen in a lake can tell us a lot about its water quality. A small amount of oxygen, up to about 14 mg/L is dissolved in water. Oxygen enters a lake from the atmosphere by wave action and from inflow streams. This dissolved oxygen is breathed by fish and zooplankton and is needed by them to survive. However, the concentration of dissolved oxygen in lakes is affected by temperature and has well-defined seasonal cycles.
During the winter months when the lake is frozen over the water below the ice equilibrates to a temperature of 4 degrees Celsius (at which temperature water is at its maximum density). Cold water can hold more dissolved oxygen than warm water. In winter and early spring, when the water temperature is still low, the DO concentration is high since no thermal gradient is established. In July the water has becomes stratified with a layer of warm water at the surface and colder water below the thermocline*. As a result the DO concentration drops significantly. By August there is very steep drop in water temperature and a well- established thermocline at 10 meters depth. Hence the DO concentration drops significantly below the thermocline since the DO at this depth can no longer be replenished. However, by October the water temperature has dropped significantly, and there is little thermocline. DO concentrations below the thermocline are quite low, but over the winter months will return to the levels seen in the early Spring. Cold water fish such as all species of trout live in the cold water below the hyperlimnion and will not survive well if DO levels fall below 4mg/L. While Otter Lake is not currently characterized as a “trout” lake, the DO levels we experience at present would indicate that the lake would support species of trout if they were introduced.
* A thermocline is the transition layer between warmer mixed water at the water’s surface and cooler deep water below. … It is relatively easy to tell when you have reached the thermocline in a body of water because there is a sudden change in temperature.
Please refer to LAKE STEWARD REPORTS to see annual reports that provide water quality results pertaining to each year.


